Abstract
Introduction: Data from the Phase II GALEN study suggest that chemo-free induction and maintenance with obinutuzumab (G), a glycoengineered type II anti-CD20 antibody, plus lenalidomide (LEN) may have favorable activity and tolerable safety in patients (pts) with relapsed/refractory (R/R) FL (Morschhauser et al. Hematol Oncol 2017). The combination of G and the anti-programmed death-ligand 1 (PD-L1) antibody atezolizumab (atezo), which has a complementary mode of action to anti-CD20 antibodies, has shown activity in R/R FL (Palomba et al. Hematol Oncol 2017). Atezo-G-LEN has the potential to enhance anti-tumor immune response in R/R FL. Interim data from a Phase Ib/II study (NCT02631577) assessing the safety and efficacy of induction and maintenance with atezo-G-LEN in pts with R/R FL are reported.
Methods: This is an ongoing, open-label, multicenter study of pts with R/R FL (excluding grade [Gr] 3b) who have received ≥1 prior anti-CD20 antibody-containing chemo-immunotherapy regimen. The study comprises an initial 3+3 dose-escalation phase (to determine the recommended Phase II dose [RP2D] of LEN for the triplet regimen) followed by an expansion phase (RP2D of LEN). Pts receive induction treatment with six 28-day cycles of: G 1000mg IV on day (D) 1, D8, and D15 of Cycle (C) 1 and D1 of C2-6; atezo 840mg IV on D1 and D15 of C2-6; and LEN 15mg or 20mg (dose escalation), or at the RP2D (expansion), PO on D1-21 of C1-6. Pts with a complete response (CR), partial response, or stable disease at the end of induction (EOI) receive 24 months of maintenance with G 1000mg on D1 every 2 months, atezo 840mg on D1 and D2 monthly, and LEN 10mg on D1-21 monthly (months 1-12). Primary endpoints are dose-limiting toxicities (DLTs) during C2, safety/tolerability, and CR (PET-CT) rate by Independent Review Committee (IRC) at EOI (modified Lugano 2014 criteria) in the LEN 20mg (RP2D) expansion cohort. Data cut-off was February 28, 2018.
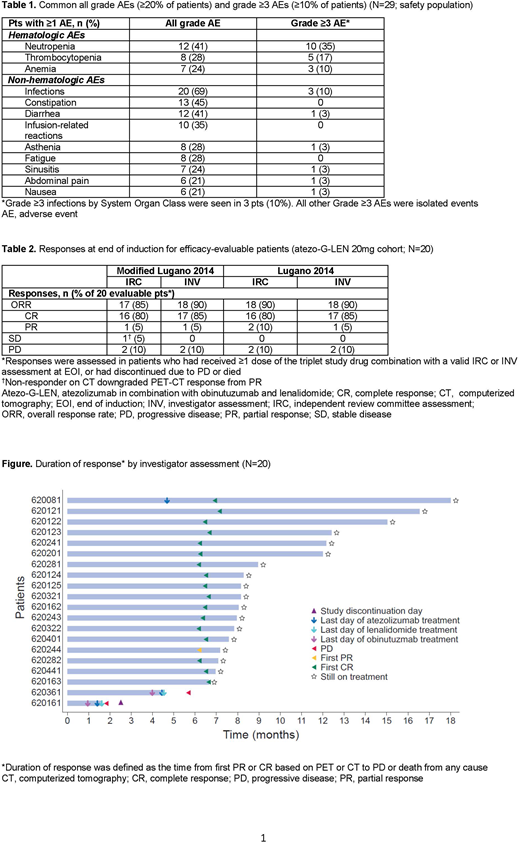
Results: At the time of interim analysis (IA), 29 pts were enrolled and treated (LEN 15mg, n=4; LEN 20mg, n=25). Three pts were receiving induction treatment, 5 had discontinued during induction (death due to progressive disease [PD], n=3; PD, n=1; withdrawal of consent, n=1), 21 had completed induction, and 20 were still receiving maintenance. Baseline characteristics at study entry were: median age, 62 years (range 38-79); male, 52%; Ann Arbor Stage III-IV, 86%; FLIPI high risk (≥3), 28%; bulky disease (≥7cm), 21%; ≥2 prior lines of therapy, 52%; refractory to last treatment line, 48%; and pts with early progression (within 24 months) on first-line treatment (POD24), 31%. No DLTs were reported during C2 at either LEN dose during dose escalation; therefore, LEN 20mg was selected as the RP2D for expansion. Median dose intensity for all 3 study drugs was 95-100% during induction and 100% during maintenance. Twenty-eight pts (97%) had ≥1 adverse event (AE): 18 (62%) Gr ≥3 AEs; 9 (31%) serious AEs; 2 (7%) AEs leading to discontinuation of any study drug (Gr 2 pneumonitis and Gr 2 myalgia secondary to myositis, both leading to discontinuation of atezo); 3 (10%) AEs leading to LEN dose reduction; and 22 (75%) AEs requiring interruption of any treatment (including LEN dose reduction, missed doses or dose delays); no Gr 5 AEs were reported. Common AEs (all-Gr and Gr ≥3) are shown in Table 1. The most common Gr ≥3 AEs were hematologic toxicities (neutropenia [35%], thrombocytopenia [17%], and anemia [10%]) and infections (10%). The most common AEs of special interest (≥4 pts) were infusion-related reactions (35%), dyspnea (21%), hyperthyroidism (17%), hypothyroidism (14%), and pyrexia (17%); all events were mild or moderate, except one case of dyspnea (Gr 3). One second primary malignancy (meningioma) was reported. Among 20 efficacy-evaluable pts, the PET-CT CR rate at EOI was 80% by IRC and 85% by investigator (modified Lugano 2014 criteria) (Table 2). All pts receiving maintenance had durable clinical responses (Figure).
Conclusions: At this IA, the overall safety profile observed for the chemo-free triplet regimen atezo-G-LEN is consistent with the known AE profiles for the individual drugs, with acceptable safety and tolerability. Response rates at EOI with atezo-G-LEN compare favorably with those reported for currently available treatments for R/R FL, with preliminary evidence of durable activity.
Salles:Amgen: Honoraria; Novartis: Consultancy, Honoraria; Morphosys: Honoraria; BMS: Honoraria, Other: Advisory Board; Epizyme: Honoraria; Servier: Honoraria, Other: Advisory Board; Celgene: Honoraria, Other: Advisory Board, Research Funding; Abbvie: Honoraria; Janssen: Honoraria, Other: Advisory Board; Takeda: Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Merck: Honoraria; Acerta: Honoraria; Servier: Honoraria; Pfizer: Honoraria; Gilead: Honoraria, Other: Advisory Board. Ghosh:PCYC: Consultancy, Research Funding, Speakers Bureau; TG Therapeutics: Honoraria, Research Funding; SGN: Consultancy, Research Funding, Speakers Bureau; F. Hoffman-La Roche Ltd: Research Funding; Spectrum: Consultancy; Forty seven Inc: Research Funding; Abbvie: Consultancy, Speakers Bureau; Juno: Consultancy, Research Funding; Genentech: Research Funding; Gilead: Consultancy, Speakers Bureau; Pharmacyclics, an Abbvie Company: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy. Lossos:Affimed: Research Funding. Palomba:Pharmacyclics: Consultancy; Celgene: Consultancy. Mehta:Incyte: Research Funding; Kite: Consultancy, Speakers Bureau; Spectrum: Consultancy; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Merck: Research Funding; Epizyme: Research Funding; Gilead: Consultancy, Speakers Bureau; Seattle Genetics: Research Funding; AstraZeneca: Consultancy, Speakers Bureau; Carevive: Other: Patient engagement; Medpage: Other: Medical website. Casasnovas:Celgene: Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; Merck: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Stevens:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Abajo:F. Hoffman-La Roche Ltd: Employment, Other: Ownership interests PLC. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Other: Ownership interests PLC. Chitra:Genentech/Roche: Employment. Wenger:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership, Other: Ownership interests PLC. Morschhauser:Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.